| World Journal of Nephrology and Urology, ISSN 1927-1239 print, 1927-1247 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Nephrol Urol and Elmer Press Inc |
| Journal website http://www.wjnu.org |
Original Article
Volume 1, Number 1, February 2012, pages 36-41
Renal Resistive Index and Urinary N-acetyl-β-glucosaminidase as Predictors of Early Renal Involvement in Patients With Essential Hypertension
Amany A Mousaa, d, Mervat M El-Eshmawya, Eman H El-Adawya, Lamiaa F Arafab, Adel El Badrawyc, Omayma M Saleha
aInternal Medicine Department, Specialized Medical Hospital, Faculty of Medicine, Mansoura University, Mansoura, Egypt
bMedical Biochemistry Department, Faculty of Medicine, Mansoura University, Mansoura, Egypt
cRadiology Department, Mansoura University Hospital, Faculty of Medicine, Mansoura University, Mansoura, Egypt
dCorresponding author: Amany A. Mousa, Specialized medical hospital, Elgomhorea st., Mansoura, Egypt
Manuscript accepted for publication January 19, 2012
Short title: Renal Resistive Index
doi: https://doi.org/10.4021/wjnu2w
| Abstract | ▴Top |
Background: The long-standing essential hypertension causes renal injury. N-acetyl-β-glucosaminidase (NAG) was considered as an indicator of the functional status of the renal tubules. The resistive index (RI), measured at the level of the interlobar arteries, is thought to reflect downstream vascular impedence and therefore, has been suggested as a measure of renal arterial stiffness. The aim of this study was to investigate whether changes in renal function assessed by urinary N-acetyl-β-glucosaminidase (NAG) and proteinurea are present in hypertensive patients with normal serum creatinine and the association of these changes with renal vascular resistance assessed by ultrasound Doppler.
Methods: The study enrolled 78 patients with essential hypertension and 57 age- and sex-matched normotensive subjects. BMI, creatinine, creatinine clearance, uric acid, lipid profile, total proteins, NAG and creatinine in urine were assessed. RI was evaluated by ultrasound Doppler of the interlobar arteries.
Results: Hypertensive patients had significantly higher urinary excreted NAG, total urinary proteins and RI than controls. RI was significantly correlated with age, systolic blood pressure, hypertension duration, total proteins, total cholesterol, excreted NAG and creatinine clearance. In multivariate analysis RI was significantly and independently influenced by NAG and creatinine clearance.
Conclusion: Early glomerular and tubular dysfunctions precede the overt changes in serum creatinine and glomerular filtration rate in hypertensive patients. Resistive index is increased along with these abnormalities. So, it should be regarded as a marker of early renal and systemic vascular damage and could help identify hypertensive patients for whom more aggressive preventive and therapeutic measures are advisable.
Keywords: Essential hypertension; N-acetyl-β-glucosaminidase (NAG); Resistive index
| Introduction | ▴Top |
The long-standing essential hypertension causes renal injury [1]. Renal dysfunction is often overlooked but relatively common condition in patients with primary hypertension. Although a slight elevation of serum creatinine is present in 3% to 10% of hypertensive patients, up to 30% of them show a mild or moderate reduction in renal function when glomerular filtration rate is estimated by more sensitive tests such as creatinine clearance [2]. It has recently been emphasized that the presence of renal dysfunction entails a cluster of unfavorable hemodynamic and metabolic changes that may accelerate the process of atherosclerosis and aggravate the global burden of cardiovascular risk [3].
The evaluation of renal vasculature and blood flow by Doppler ultrasonography is performed in hypertensive patients with increasing frequency [4]. The resistive index (RI), measured at the level of the interlobar arteries, is thought to reflect downstream vascular impedence and therefore, has been suggested as a measure of renal arterial stiffness [5]. Increased RI has been shown to correlate with the severity of renal damage in primary renal diseases [6] and to predict the rate of progression to end-stage renal disease [6, 7].
N-acetyl-β-glucosaminidase (NAG), is a lysosomal enzyme normally excreted in low amounts in the urine as a consequence of the physiological exocytosis process in the proximal tubular cells, but is excreted in abnormally high amounts in many situations characterized by exposure of these cells to various toxic substances, and it is therefore an indicator of the functional status of the renal tubules as well as tubular damage [8].
There is no doubt, however, that other factors besides elevated blood pressure contribute to the development and progression of hypertensive nephropathy (HN) [9].
The aim of this study was to detect early changes in renal function assessed by urinary NAG and presence of proteinurea in hypertensive patients with normal serum creatinine and the association of these changes with renal vascular resistance assessed by ultrasound Doppler.
| Methods | ▴Top |
The study comprised 78 patients with essential hypertension (30 males and 48 females) and 57 age- and sex-matched normotensive subjects. The hypertensive patients were attending the out patient clinic at Mansoura Specialized Medical Hospital. All subjects signed an informed consent to be included in our study. The study was approved by the local ethical committee.
The main inclusion criteria were as that: patients with essential hypertension with duration not less than five years and normal serum creatinine. Only subjects whose antihypertensive regimens were not modified in the past 6 months were included. Exclusion criteria included the presence of chronic pyelonephritis, primary glomerulonephritis, and peripheral arterial disease. Also the presence of neoplasia, hepatic or chronic heart failure (New York Heart Association class III and IV), positive history or clinical signs of ischemic heart disease, diabetes mellitus and morbid obesity.
Diagnosis of essential hypertension was made by the attending physician after complete medical history and physical examination. Routine biochemical analyses of blood and urine (including urine sediment evaluation) were carried out for each patient. Further investigation was performed only when abnormalities were found in these analyses, or when other symptoms or signs suggesting secondary hypertension were present. On the study day, after an overnight fast, height and weight were measured and venous blood was drawn to carry out routine blood tests. Blood pressure was measured with the patient in the sitting position after a 5-min rest, with a mercury sphygmomanometer (cuff size 12.5 by 40 cm). The systolic and diastolic blood pressure was read to the nearest 2 mmHg. Disappearance of Korotkoff’s sounds (phase V) was the criterion for diastolic BP. The lowest of three consecutive readings were recorded. Body mass index (BMI) was calculated by the formula: weight (in kilograms)/height (in meters squared). Creatinine, uric acid, total cholesterol (TC), triglyceride (TG), high density lipoprotein cholesterol (HDL-c), low density lipoprotein cholesterol (LDL-c) and other standard blood chemistry evaluations were performed on the serum, according to routine methods.
Second morning urine sample were obtained. Total urinary proteins were measured by Coomassie blue method as mg/L and urinary creatinine were measured automatically on a HITACHI 717 Instrument (Boehringer, Mannheim, Germany) by the Jaffe’ method without deproteinization as g/L. Urinary proteins excretion was expressed as mg/g urinary creatinine. For the determination of NAG in the second morning urine samples, a colourimetric assay was performed using 3-cresolsulfonphthaleyn-N-acetyl-β-Dglucosaminide which is hydrolysed by NAG with the release of 3-cresolsulfonphthaleyn sodium salt, which is measured photometrically at 580 nm on a HITACHI 717 Instrument; the results were expressed in units/ gram of urinary creatinine (U/gm). Creatinine clearance was estimated by the Cockcroft-Gault formula [10].
Renal ultrasonography was performed as reported in Pontremoli et al. [11]. Doppler signals were obtained from the interlobar arteries by placing the sample volume at the edge of the medullary pyramids. Mean RI ((Peak systolic velocity _ End-diastolic velocity)/ Peak systolic velocity) was calculated by using six measurements (three from each of the two kidneys) taken for each patient. The ultrasound examination of the kidneys and pulsed Doppler of the intrarenal arteries were performed using a Hitachi AU 450 machine (Tokyo, Japan) with a 3.5-MHz transducer working at 2.5 MHz for Doppler analysis.
Statistical analysis
Data were analyzed using SPSS statistical package version 10 (SPSS, Inc., Chicago, IL, USA). The quantitative data were presented as a mean and a standard deviation. For the qualitative data, Student t-test was used to compare between two groups. Simple regression analysis was performed with urinary NAG, total urinary proteins and RI as the dependent variables and all other parameters as independent variables. Multiple regression analysis to assess the independent effect of studied variables on RI was also performed. P value of < 0.05 indicates significant results.
| Results | ▴Top |
Baseline characteristics of the hypertensive patients and healthy controls are given in Table 1. Hypertensive patients had significantly higher BMI, systolic, diastolic blood pressure (P < 0.001), serum uric acid, urinary NAG (P < 0.001), total proteins, TC, TG, LDL-c and RI (P < 0.001), creatinine clearance was significantly lower compared to normal controls (P = 0.001).
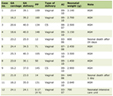 Click to view | Table 1. Clinical and Biochemical Characteristics of Hypertensive Patients and Healthy Controls |
Table 2 showed urivariate correlation of urinary NAG, total proteinurea and RI with clinical and biochemical variables. There was a statistically significant correlations between urinary NAG excretion and age, systolic blood pressure, duration of hypertension, serum uric acid, total urinary proteins and RI (P = 0.001). Total urinary proteins were positively correlated with systolic blood pressure, duration of hypertension, serum uric acid and resistive index (0.014). Resistive index was significantly correlated with age, systolic blood pressure, hypertension duration, TC and creatinine clearance.
 Click to view | Table 2. Univariate Correlation of Urinary NAG, Total Proteins and Resistive Index With Clinical and Biochemical Variables in Hypertensive Patients |
A multiple regression analysis to assess the independent effect of studied variables on RI was also performed (Table 3). RI was significantly and independently influenced by age, systolic blood pressure, Urinary NAG (P = 0.003) and creatinine clearance.
 Click to view | Table 3. Multiple Regression Analysis of Resistive Index and Selected Variables |
| Discussion | ▴Top |
The kidney plays a dual role in primary hypertension [12]. On the one hand, it is thought to be the cause of blood pressure elevation; on the other, it may suffer from the long-term negative consequences of the hypertensive state. Furthermore, renal function may serve as a sensor of cardiovascular risk, since it is inversely related to the occurrence of cardiovascular events. The incidence of cardiovascular disease progressively increases as glomerular filtration rate deteriorates, reaching dramatically high levels in patients on renal replacement therapy [13].
In the present study, the urinary NAG excretion was significantly elevated in hypertensive patients compared to control subjects. Similar findings were reported by Tylicki et al. [14] and Kretowicz et al. [15]. In addition, the elevated urinary NAG excretion had statistically significant univariate correlations with patient's age, duration of hypertension, systolic blood pressure and serum uric acid. Tylicki et al. [9] found statistically significant correlations between urinary NAG excretion and age, plasma level of uric acid, and HOMA-IR index.
It has long been known that overt proteinuria is a strong independent risk factor for cardiovascular disease both in diabetic and nondiabetic populations [16]. The total urinary protein in our study was significantly higher in patients than healthy controls and was significantly correlated with duration of hypertension, systolic blood pressure, serum uric acid and urinary NAG excretion. So, the abnormalities in both urinary NAG excretion and proteinuria were correlated with a cluster of unfavorable abnormalities like old age, longstanding hypertension, higher systolic blood pressure and uric acid.
In our study the serum creatinine of the hypertensive patients was normal; Leoncini et al. [17] stated that serum creatinine, is a less accurate indicator of renal function as compared with creatinine clearance, since the latter takes into account age, gender, and body weight, variables that are known to influence glomerular filtration rate. Although the estimated creatinine clearance in our patients was found to be significantly lower than normal subjects, yet within the normal range. So, our findings support the fact that the presence of normal creatinine clearance would not exclude the beginning of renal affection in hypertensive subjects.
BMI was significantly higher than in controls; however, it had no significant correlations with the assessed variables for renal dysfunction. This finding seems to be in contrast with other evidence that highlighted the importance of obesity as risk factor for end-stage renal disease [18]. However this evidence arises chiefly from longitudinal studies including severely obese subjects. In our study, patients with BMI greater than 35 kg/m2 were excluded to reduce the potential influence of the glomerulopathy related to an increased BMI and especially to severe degrees of obesity. Moreover, the Framingham Offspring Study showed that obesity was not associated per se with an independent risk to develop stage 3 chronic kidney disease, after adjustment for known CV disease risk factors [19].
The clinical relevance of renal doppler indices has been discussed equivocally. Our results showed a significant elevation of RI measured at the level of interlober arteries in hypersensitive patients compared to controls. Some authors relate these Doppler indices to damage of renal vascular bed (nephroscleosis). However, others relate them to alteration of the arterial system [20]. The increased RI was significantly correlated with patient's age, systolic blood pressure, hypertension duration, total cholesterol. Taniwaki et al. [21] reported that RI in patients with diabetic nephropathy was significantly correlated with age, duration of diabetes, systolic and diastolic blood pressure. These data indicate that renal RI could reflect systemic vascular damage. Moreover, Ohta et al. [22] were able to show that RI correlated with carotid artery structure and elasticity in patients with essential hypertension and explained it by the systemic arterial disease with impairment of the mechanical properties of large artery walls in these patients.
On the other hand, there was a significant correlation between the increased RI in our study and creatinine clearance, urinary NAG and total proteins excretion. Moreover, multivariate regression analysis showed that the associations with creatinine clearance and urinary NAG were independent of other variables like age, blood pressure or serum cholesterol. Similarly, Derchi et al. [23] reported an increase in intra renal vascular stiffness may occur together with the impairment of renal function independent of several potential confounders such as age and blood pressure level. Others demonstrated that RI ≥ 0.8 is an even greater independent predictor of renal disease progression than clinical proteinurea and glomerular filtration rate reduction [24]. Boddi et al. [25] assessed renal tubular function and RI in hypertensive and normotensive patients with tubulointerstilial nephropathy and concluded than RI measurement allows the early identification of signs of tubular dysfunction when renal function is still preserved. Recently, other study identified overt proteinurea (≥ 1g/gm creatinine), RI (> 0.7) and high systolic pressure (≥ 140 mmHg) as independent predictors of worsening renal function [26].
Conclusion
Early glomerular and tubular dysfunctions precede the overt changes in serum creatinine and glomerular filtration rate. Resistive index is increased along with these abnormalities in hypertensive patients. So, it should be regarded as a marker of early renal and systemic vascular damage and could help identify hypertensive patients for whom more aggressive preventive and therapeutic measures are advisable.
Conflict of Interest
Authors disclose no sponsorship or funding arrangements relating to their research and all authors disclose no possible conflicts of interest.
| References | ▴Top |
- Rutkowski B, Tylicki L, Manitius J, Lysiak-Szydlowska W. Hypertensive nephropathy - an increasing clinical problem. Miner Electrolyte Metab. 1999;25(1-2):65-68.
pubmed doi - Ruilope LM. The kidney as a sensor of cardiovascular risk in essential hypertension. J Am Soc Nephrol. 2002;13 Suppl 3:S165-168.
pubmed - Perticone F, Maio R, Tripepi G, Zoccali C. Endothelial dysfunction and mild renal insufficiency in essential hypertension. Circulation. 2004;110(7):821-825.
pubmed doi - Tublin ME, Bude RO, Platt JF. Review. The resistive index in renal Doppler sonography: where do we stand? AJR Am J Roentgenol. 2003;180(4):885-892.
pubmed - Mostbeck GH, Kain R, Mallek R, Derfler K, Walter R, Havelec L, Tscholakoff D. Duplex Doppler sonography in renal parenchymal disease. Histopathologic correlation. J Ultrasound Med. 1991;10(4):189-194.
pubmed - Sugiura T, Nakamori A, Wada A, Fukuhara Y. Evaluation of tubulointerstitial injury by Doppler ultrasonography in glomerular diseases. Clin Nephrol. 2004;61(2):119-126.
pubmed - Splendiani G, Parolini C, Fortunato L, Sturniolo A, Costanzi S. Resistive index in chronic nephropathies: predictive value of renal outcome. Clin Nephrol. 2002;57(1):45-50.
pubmed - Bazzi C, Petrini C, Rizza V, Arrigo G, Napodano P, Paparella M, D'Amico G. Urinary N-acetyl-beta-glucosaminidase excretion is a marker of tubular cell dysfunction and a predictor of outcome in primary glomerulonephritis. Nephrol Dial Transplant. 2002;17(11):1890-1896.
pubmed doi - Tylicki L, Rutkowski B, Horl WH. Multifactorial determination of hypertensive nephroangiosclerosis. Kidney Blood Press Res. 2002;25(6):341-353.
pubmed doi - Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31-41.
pubmed doi - Pontremoli R, Viazzi F, Martinoli C, Ravera M, Nicolella C, Berruti V, Leoncini G, et al. Increased renal resistive index in patients with essential hypertension: a marker of target organ damage. Nephrol Dial Transplant. 1999;14(2):360-365.
pubmed doi - Ruilope LM, Campo C, Rodriguez-Artalejo F, Lahera V, Garcia-Robles R, Rodicio JL. Blood pressure and renal function: therapeutic implications. J Hypertens. 1996;14(11):1259-1263.
pubmed doi - Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32(5 Suppl 3):S112-119.
pubmed doi - Tylicki L, Puttinger H, Rutkowski P, Nieweglowski T, Rutkowski B, Horl WH. Multifactoral analysis of determinators for renal injury in essential hypertension. J Hum Hypertens. 2006;20(1):93-95.
pubmed doi - Kretowicz M, Ukleja-Adamowicz M, strozecki P, Buczkowski K, klucz K, Paczuski R, et al. PAI-1 and hyperuricaemia: another face of endothelium dysfunction in essential hypertension. Endothelium, hypertension, metabolism 2005; 9:261-265.
- Miettinen H, Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Proteinuria predicts stroke and other atherosclerotic vascular disease events in nondiabetic and non-insulin-dependent diabetic subjects. Stroke.1996; 27:2033–2039.
- Leoncini G, Viazzi F, Parodi D, Vettoretti S, Ratto E, Ravera M, Tomolillo C, et al. Mild renal dysfunction and subclinical cardiovascular damage in primary hypertension. Hypertension. 2003;42(1):14-18.
pubmed doi - Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144(1):21-28.
pubmed - Foster MC, Hwang SJ, Larson MG, Lichtman JH, Parikh NI, Vasan RS, Levy D, et al. Overweight, obesity, and the development of stage 3 CKD: the Framingham Heart Study. Am J Kidney Dis. 2008;52(1):39-48.
pubmed doi - Hausberg M, Lang D, Barenbrock M, Kosch M. What do Doppler indices of renal perfusion tell us for the evaluation of renal disease? J Hypertens. 2005;23(10):1795-1797.
pubmed doi - Taniwaki H, Ishimura E, Matsumoto N, Emoto M, Inaba M, Nishizawa Y. Relations between ACE gene and ecNOS gene polymorphisms and resistive index in type 2 diabetic patients with nephropathy. Diabetes Care. 2001;24(9):1653-1660.
pubmed doi - Ohta Y, Fujii K, Arima H, Matsumura K, Tsuchihashi T, Tokumoto M, Tsuruya K, et al. Increased renal resistive index in atherosclerosis and diabetic nephropathy assessed by Doppler sonography. J Hypertens. 2005;23(10):1905-1911.
pubmed doi - Derchi LE, Leoncini G, Parodi D, Viazzi F, Martinoli C, Ratto E, Vettoretti S, et al. Mild renal dysfunction and renal vascular resistance in primary hypertension. Am J Hypertens. 2005;18(7):966-971.
pubmed doi - Radermacher J, Ellis S, Haller H. Renal resistance index and progression of renal disease. Hypertension. 2002;39(2 Pt 2):699-703.
pubmed doi - Boddi M, Cecioni I, Poggesi L, Fiorentino F, Olianti K, Berardino S, La Cava G, et al. Renal resistive index early detects chronic tubulointerstitial nephropathy in normo- and hypertensive patients. Am J Nephrol. 2006;26(1):16-21.
pubmed doi - Sugiura T, Wada A. Resistive index predicts renal prognosis in chronic kidney disease. Nephrol Dial Transplant. 2009;24(9):2780-2785.
pubmed doi
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Nephrology and Urology is published by Elmer Press Inc.
