| World Journal of Nephrology and Urology, ISSN 1927-1239 print, 1927-1247 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Nephrol Urol and Elmer Press Inc |
| Journal website https://www.wjnu.org |
Original Article
Volume 11, Number 1, June 2022, pages 24-30
Clinicopathological Characteristics of Drug-Induced Acute Interstitial Nephritis and Role of Steroids in Management: A Single-Center Observational Study
Ayesha Aziza, Sonia Yaquba, c , Safia Awana, Amna Khalidb, Muhammad Raheel Abdul Razzaquea
aSection of Nephrology, Department of Medicine, Aga Khan University Hospital, Karachi, Pakistan
bAga Khan University Medical College, Karachi, Pakistan
cCorresponding Author: Sonia Yaqub, Section of Nephrology, Department of Medicine, Aga Khan University Hospital, PO Box 3500, Stadium Road, Karachi, Pakistan
Manuscript submitted July 12, 2021, accepted December 3, 2021, published online March 12, 2022
Short title: Steroids in Acute Interstitial Nephritis
doi: https://doi.org/10.14740/wjnu427
| Abstract | ▴Top |
Background: Acute interstitial nephritis (AIN) is a potentially reversible, but underdiagnosed cause of acute kidney injury. The role of steroids in the management of drug-induced AIN is debatable. There is scarcity of data on clinical characteristics of drug-induced AIN, management approach and effect of steroids on renal outcomes from the low-middle-income countries.
Methods: We performed a retrospective study of patients with biopsy-proven AIN over a period of 12 years. The main outcomes were recovery of renal function (early (≤ 3 weeks) or late (> 3 weeks)) and hemodialysis (HD) dependence at 12 weeks.
Results: A total of 48 (4.9%) AIN cases were found among 978 renal biopsies. Mean age was 47.6 ± 12.1 years and 56.3% were males. The offending agent could be identified in half of the patients (non-steroidal anti-inflammatory drugs (NSAIDs) most commonly followed by antibiotics, diuretics and proton pump inhibitor (PPI)). Three quarters of patients presented with estimated glomerular filtration rate (eGFR) < 15 mL/min/1.73 m2 (n = 36), out of which 58.3% (n = 21) required HD. Thirty-nine (81.3%) patients ended up receiving steroids. Mean dose of prednisone was 0.8 ± 0.2 mg/kg per day. Early use of steroids (≤ 11 days) was significantly associated with earlier (≤ 21 days) onset of recovery (P = 0.003) as compared to late use of steroids (> 11 days).
Conclusion: Our data showed the benefit of earlier use of steroids in achieving rapid and complete renal recovery in drug-induced AIN in a low-middle-income country with frequent use of over-the-counter drugs. Late steroid use (≥ 3 weeks) was not associated with any further recovery at an additional risk of exposing patients to undue adverse effects.
Keywords: Acute interstitial nephritis; Drug-induced AIN; Steroids; Kidney biopsy
| Introduction | ▴Top |
Acute interstitial nephritis (AIN) is a common and mostly reversible cause of acute kidney injury (AKI), yet it remains relatively underdiagnosed. The incidence of AIN averages at 2.8%, ranging from 1% to 10% of all biopsies as calculated from the reports of the large kidney biopsy registries [1]. A large albeit retrospective study [2] demonstrated that when analysis is restricted to biopsies performed for patients with AKI, the prevalence increases to 13%. Drugs account for 70-75% of cases. The remaining causes are associated with autoimmune disease, infection or idiopathic. Early diagnosis, identification and withdrawal of offending agent are key factors in preserving renal function and ensure good long-term renal prognosis. Renal recovery is variable as renal function may return to baseline or stabilize with residual damage, after discontinuation of the offending agent or treatment of the causative systemic illness and may also progress despite corticosteroids and other supportive management. The role of steroids in the treatment in drug-induced AIN remains to be established. Whereas in some studies, steroids have shown to improve outcomes and accelerate renal recovery, others have reported no beneficial effect on the rate or extent of recovery of kidney function [3]. This is more relevant in low-middle-income countries (LMICs), like Pakistan, with weak and unorganized health care system, inadequate nephrology workforce and scarce availability of dialysis and transplant facilities, thus imposing financial distress on the population and resulting in poor outcomes. In the present study, we aimed to determine the causes and clinical presentation of AIN as well as time of initiation of steroids, effect of steroids on recovery and time to renal recovery in our population.
| Materials and Methods | ▴Top |
Study population
We performed a retrospective review of medical records of native renal biopsies performed at our tertiary care center, Aga Khan University Hospital, over the course of 12 years from January 2007 to December 2018. Forty-eight patients were identified with AIN meeting the inclusion criteria and a detailed medical chart review was undertaken. This study was approved by the Institutional Review Board of the Ethical Committee at the Aga Khan University Hospital (approval no. 2019-1195-3019) and was conducted in accordance with the principles of the Declaration of Helsinki as revised in 2013.
This study is based upon review of medical records only and does not contain any studies with human participants or animals performed by any of the authors.
We included all patients aged 18 years or older with biopsy-proven AIN with known or unknown offending drug agent. All patients with inconclusive biopsy specimen, graft biopsies after renal transplant, chronic tubulointerstitial nephritis or acute tubular necrosis on biopsy, or primary glomerular pathology with secondary acute interstitial nephritis were excluded.
Diagnosis of AIN
All kidney biopsy specimens were processed for light microscopy and immunofluorescence and interpreted by the consultant renal histopathologist at our hospital. Diagnosis of AIN was made if tubulitis with or without focal tubular atrophy, and interstitial inflammatory infiltrate was found with varying degrees of edema or fibrosis. Severity of AIN was graded by consultant histopathologist based on diffuse vs. patchy involvement, variable severity of interstitial infiltrate and degree of tubulitis and acute tubular injury. Electron microscopy was not performed as the facility was not available at our hospital.
Clinical data
Medical charts and electronic laboratory data were reviewed and following details were recorded: demographics, serum creatinine (SCr) and estimated glomerular filtration rate (eGFR) at presentation, need for hemodialysis (HD), presenting symptoms, urinalysis, protein quantification as measured by 24-h urinary protein (g/day) or spot protein to creatinine ratio (PCR) wherever available, offending agent or history of infection or autoimmune disease if any, steroid dose, time to initiation of steroids and total duration, time to initial recovery of renal function , eGFR and HD dependence at 12 weeks from presentation. eGFR was calculated by CKD-EPI-PAK equation. Baseline SCr was defined as the lowest value of SCr available in the previous 1 year before presentation.
Exposure and outcomes
The management approach was studied for all patients. The decision to prescribe steroids as well as the period of observation was left on discretion of the treating physicians. In patients who were given steroids, the exposure was defined as the use of steroids and subgroup analysis was performed to ascertain the effects of steroid dose, duration and time to commencement on outcomes if any.
The main outcomes were: 1) recovery of renal function, either early (defined as beginning of downtrend of SCr ≤ 3 weeks) or late (> 3 weeks); 2) complete recovery (SCr recovery to within 25% of baseline or SCr < 1.4 mg/dL if baseline not available) or partial recovery (≥ 25% improvement in SCr but not meeting the criterion for complete recovery) or no recovery (< 25% improvement in SCr or HD dependence) at 12 weeks from presentation; and 3) HD dependence at 12 weeks’ follow-up or at the time of death (if before 12 weeks).
Statistical analysis
Data were analyzed using SPSS 23.0 for Windows (SPSS Inc., Chicago, IL, USA). Results are expressed as mean ± standard deviation (SD) for continuous variables wherever normal distribution could be assumed. If the data were not normally distributed, median values with interquartile ranges (IQRs) were reported. Categorical variables are presented as percentages. Qualitative variables were analyzed by Fisher’s exact and Chi-square tests. Statistical significance was defined as P < 0.05.
| Results | ▴Top |
Demographics
Table 1 lists demographic and clinical characteristics of patients with AIN at presentation. Overall, 48 (4.9%) AIN cases were found among 978 renal biopsies performed during the study period. Mean age was 47.6 ± 12.1 years, and 56.3% were males. Almost one-third of the patients had diabetes. The offending agent was identified in 45.8% cases with non-steroidal anti-inflammatory drugs (NSAIDs, 45.4%) being the most common, followed by antibiotics (22.7%), diuretics (9%), proton pump inhibitors (PPIs, 9%), rifampicin (4.5%), allopurinol (4.5%) and homeopathic medications (4.5%). History of infection was found in 6.25% (n = 3). One patient was diagnosed with Sjogren syndrome. Mean 24-h urinary protein was 1.75 ± 0.96 g/day with four patients (8.3%) having nephrotic range proteinuria. Microscopic hematuria was found in 54.2% and leukocyturia in 58.3% patients respectively.
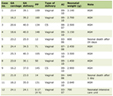 Click to view | Table 1. Characteristics of Study Population |
Half of the patients had baseline SCr available (median Cr = 1.0 mg/dL), with baseline eGFR of ≥ 60 mL/min/1.73 m2 in 83.3% cases and between 30 and 60 mL/min/1.73 m2 in 12.6% cases. Thirty-six (75%) patients had eGFR < 15 mL/min/1.73 m2 at presentation and 58.3% (n = 21) required HD. Most of the patients showed moderate to severe AIN on renal histopathology (60.41%), while 16.7% had severe AIN and 22.9% had mild AIN. Histological findings, however, did not correlate with the severity of renal dysfunction at presentation.
Management approaches and steroid use
A total of 39 patients (81.3%) ended up receiving steroids, out of which 64.1% were started on steroids after an initial period of observation (range: 7 - 70 days). Mean time to steroid commencement was 14 ± 17.39 days.
Two groups were defined: early steroids group (commenced steroids in ≤ 11 days of diagnosis) and late steroids (commenced steroids > 11 days after diagnosis). For three patients, time to steroid initiation was not known.
Outcomes
Renal recovery was documented in 29 (60.4%) patients, of which 14 (29.2%) patients made full recovery and 15 patients (31.3%) made partial recovery. Nineteen patients (39.5%) did not recover, and nine patients (47.4%) remained HD dependent including two patients who died during the follow-up period. No difference was made by steroid use among patients who needed HD from the outset (P = 0.971). Older age, co-morbidities, AIN severity, and eGFR at presentation did not significantly alter the chances of recovery on further analysis (Table 2). Need for HD at presentation emerged as the worst prognostic factor with 34.5% showing any recovery versus 65.5% who did not require HD (P = 0.220).
 Click to view | Table 2. Factors Affecting Recovery of Renal Function |
Mean dose of prednisone was 0.8 ± 0.2 mg/kg per day. Median of total duration of treatment including the tapering period was 9 weeks (IQR: 4 - 16). Early use of steroids was significantly associated with recovery of renal function (complete or partial, P = 0.007) as well as earlier recovery (P = 0.003) as compared to late steroids (Fig. 1). Start of earlier recovery (≤ 3 weeks) of renal function was significantly associated with complete recovery on follow-up (P = 0.003). A trend towards complete recovery was observed in early steroid group (64.3%, n = 9) vs. “late steroids” (25%, n = 1) group; however, the difference did not reach statistical significance (P = 0.163). No significant correlation between the low dose (0.5 mg/kg/day) and high dose (1 mg/kg/day) steroids was found in terms of renal recovery.
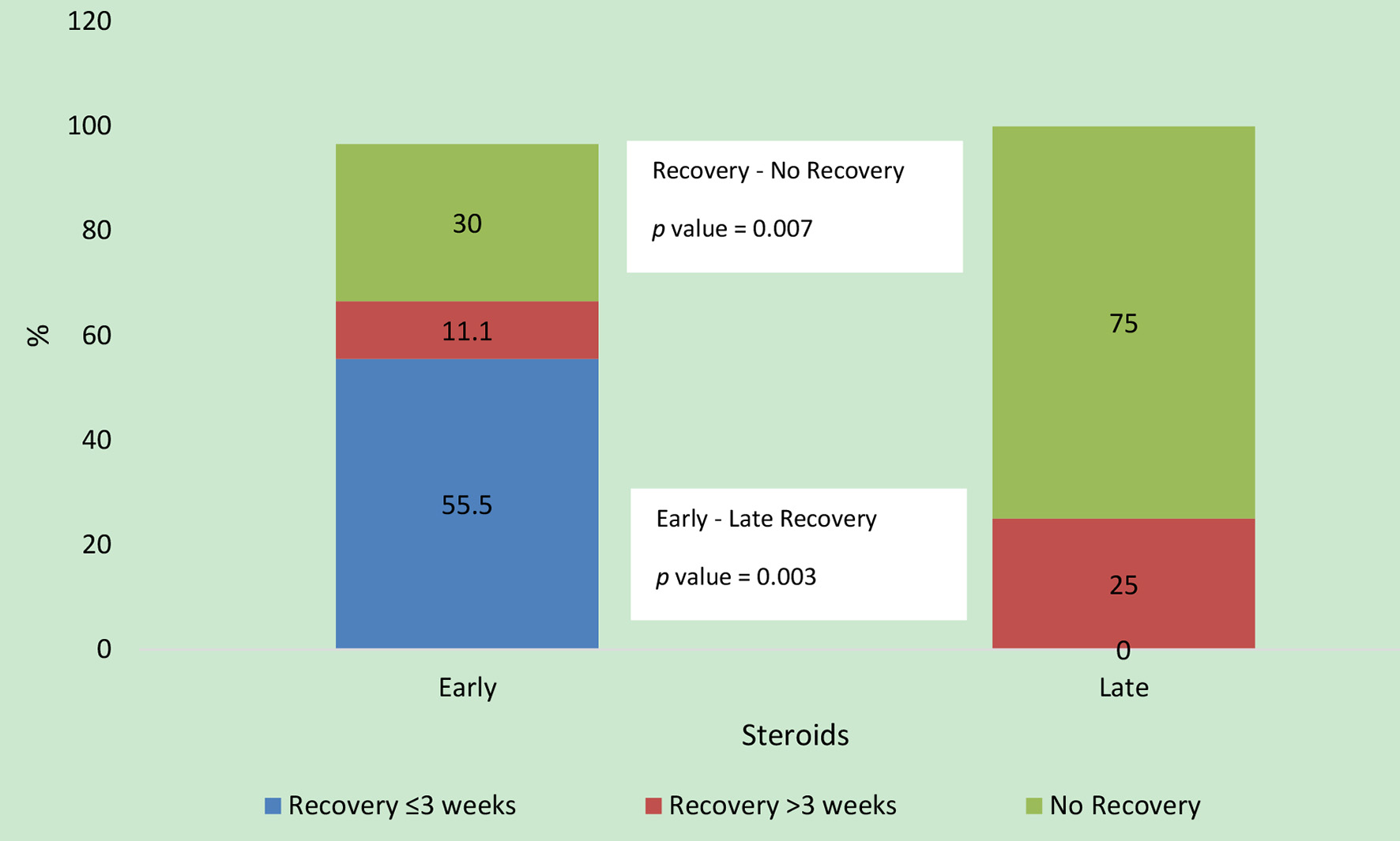 Click for large image | Figure 1. Comparison of outcomes between the two steroid groups. Of the 20 patients, 14 (70%) patients recovered in early steroid group and four (25%) patients recovered in the late steroid group. Time to recovery data were missing for two patients in the early steroid group. |
Patients with moderate to severe AIN were more likely to remain dialysis dependent (P = 0.09). Presence of microscopic hematuria and oliguria at presentation were significantly associated with need of initiation of HD (P = 0.02 and 0.015, respectively). No other risk factors for need of HD could be ascertained, although more males (71.4%) than females (28.6%) required HD (P = 0.062).
| Discussion | ▴Top |
Given the adverse effects of AKI and its severity and reversibility on the renal prognosis, AIN is increasingly being recognized as a significant, potentially reversible cause of AKI. The rise in prevalence of AIN could be accounted for by polypharmacy, inevitable in an era of multiple co-morbid conditions with increasing life expectancy. Virtually any drug can cause AIN but NSAIDs and antimicrobials top the list followed by diuretics, cimetidine and PPIs. It is difficult to ascertain the exact etiology in patients who are on multiple medications and may not recall the addition of a new agent. Poor prognosis has been linked to longer duration of exposure to the inciting agent and delay in initiating treatment with steroids [3]. This is more relevant in LMICs, like Pakistan, with suboptimal extent and quality of health care delivery and ubiquitous use of traditional medicine for primary health care as well as over-the-counter availability of prescription drugs.
Frequency of AIN in our study (4.9%) is higher than previously recorded by two single-center studies from Pakistan and India both analyzing biopsy data till 2008 (1.1%) [4, 5]. In a larger (1979 - 2002 data) single-center cohort from China, prevalence was found to be similar (1.1%) [6]. Nevertheless, our findings are similar to studies analyzing more recent data (4.4%: 2000 - 2015, 4.7%: 2000 - 2012) [7, 8]. In another recently published study, analyzing Spanish registry data (1994 - 2009), a rise in AIN cases of 4.2% was observed in the last 4 years (2006 - 2009), which was even more striking in the elderly (> 12%), with an overall prevalence of 2.7% [2]. Although these findings may reflect the changing patterns in our attitude towards renal biopsy in patients, specially elderly, with suspected AIN, evidence is piling up for an actual increase in prevalence over recent years [7-9]. Epidemiological data are needed to ascertain what exactly is driving this rise in AIN cases.
Presentation of AIN was also unusual as majority of patients in our cohort presented with eGFR < 15 mL/min/1.73 m2, more than half of whom required HD (n = 21) which is usually not the case in AIN and is almost twice as high as reported in other studies [10]. Although, average duration of exposure to offending agents could not be determined, this markedly severe presentation could reflect a prolonged use of inciting agents due to delays in recognition and referral to nephrologists. The tendency to prefer self-treatment, reliance on informal and unqualified practitioners, and a poor referral system prevailing in LMIC are reasons for a delayed presentation to tertiary care centers.
Drug-induced AIN predominates the etiological diagnosis but there has been a shift in the type of drug with NSAIDs and PPIs being increasingly recognized as the main culprit and antibiotics, less and less implicated [11-15]. We found NSAIDs to be the most common cause followed by antibiotics. Another study from Pakistan also found NSAIDs to be the most common followed by contrast agents and anti-tuberculosis therapy [16]. Perazella et al found NSAIDs to be the commonest followed by PPIs [14]. The huge and largely uncontrolled consumption of NSAIDs and antibiotics among the general population, particularly in elderly, calls for more effective drug regulatory measures and development of check and balance for over-the-counter availability of drugs. Moreover, there is a need to create awareness among general population to avoid self-medication and to limit the use of medications except when clearly indicated under the treating clinician’s close supervision. Infection-related causes could still be relevant in a developing country like ours, although prevalence in our cohort was relatively low (6.25%) similar to overall reported incidence of 4-10%. We could not ascertain the cause of AIN in most patients (45.8%) due to the tendency to self-medicate among masses, use of traditional/herbal medication and more importantly poly-medication and therefore being unable to recall addition of a new agent. A few patients (n = 4) presented with nephrotic range proteinuria which could be explained by NSAID-induced minimal change disease in three of them where history of intake was available. However, that could not be ascertained due to non-availability of electron microscopy. Moreover, 16 patients (33.3%) had diabetes mellitus and might have had underlying diabetic nephropathy that may have accounted for nephrotic range proteinuria.
AIN is considered potentially reversible, yet it is interesting to note that recovery of renal function is often incomplete. Historically, it was considered relatively benign in view of, albeit delayed, but often complete recovery in methicillin-induced AIN but recent studies showed partial recovery in as much as 40% of patients [17]. Permanent renal insufficiency has been reported in up to 56% patients with NSAID-induced AIN [15]. Data on the development of end-stage renal disease (ESRD) are scarce. Of the patients, 10-12.5% remain HD dependent [18, 19]. None of the patients, who did not require HD in first 12 months, developed ESRD over a mean follow-up of > 5 years [20]. In elderly, the median time for patients with a histological diagnosis of AIN to either reach ESRD or die was 8.63 years (range 5.34 - 8.63) [21]. Percentage of complete renal recovery varies markedly in different cohorts (49-69%) [10, 15, 18]. We observed even poorer outcomes with only 29.2% showing complete recovery and 18.8% remaining on HD. This likely reflects the delay in diagnosis and longer exposure to offending agent, which in turn is related to delayed presentation, the access to healthcare facilities and awareness. It may also be due to a smaller number of patients having allergic type AIN on histopathology in our cohort. Baseline creatinine was not available for 14 out of 19 patients who failed to recover; hence, role of chronic renal dysfunction cannot be entirely ruled out. Another study from Pakistan reported renal recovery in 71.61% cases but only 37.4% patients had biopsy-proven AIN [16].
In the absence of a randomized trial, steroid use, dose, timing and duration have been debated for decades due to conflicting results from various observational, uncontrolled studies [1, 10, 20]. We demonstrated a low threshold for using steroids in our population as most of the patients ended up receiving steroids either as the first-line therapy or in case of no or partial recovery after a period of observation. It has been previously shown that those who received steroid treatment within first 2 weeks after withdrawal of offending agent were more likely to return to baseline renal function and delay was associated with progression of interstitial fibrosis [10, 22]. Given the scarcely available renal replacement therapy or transplant facilities, and huge economic challenges posed by the loss of productivity adjusted life years, these results explain the tendency for use of steroids in our cohort besides an unusually severe presentation. Our data suggested that early initiation of steroids significantly correlated with better short-term renal prognosis with rapid recovery and higher probability of reaching baseline. Delayed steroid initiation did not improve any outcomes at the added risk of exposing patients to undue side effects. Low-dose steroid (0.5 mg/kg) was as effective as the high dose in inducing remission, hence there was no added benefit of high-dose steroids.
We also found an association between early initiation of recovery and greater probability of return to baseline. Hence, timing of steroid treatment is crucial as we may lose the critical period where ongoing inflammation leads to significant tubular atrophy and fibrosis diminishing the chances of complete recovery. Although our finding corroborates the results obtained by recently published larger observational studies on timing and duration of treatment with steroids [22, 23], we could not demonstrate any statistical superiority of steroids over conservative management due to disproportionately low number in conservative strategy group. Prospective, randomized studies are needed to determine the optimal duration of observation and starting steroids.
Increased interstitial fibrosis/tubular atrophy on histopathology and smaller kidney size on ultrasound have been linked to poor response to steroids [10]. We did not find any correlation between histopathological severity of AIN and response to steroids or renal outcomes. Many other prognostic markers have been studied but duration of AKI and length of exposure have been most convincing in view of consistent reproducibility across studies [15, 19]. Need for HD at presentation emerged as the worst prognostic factor in our cohort and no difference was noted with addition of steroids.
Our finding suggests that with moderate to severe renal dysfunction not requiring HD at the outset, steroids have a beneficial role in accelerating renal recovery. Although there is a trend towards early steroid administration in patients requiring HD, it does not appear to improve outcomes for this population. Prendecki et al reported better long-term outcomes in AIN treated with steroids including fewer patients on HD on follow-up but they also could not demonstrate statistically significant superiority of steroids over conservative measures in terms of better recovery after initial dependence on HD [24].
Limitations
Retrospective settings and short duration of follow-up are the main limitations of our study. Moreover, the sample size of our study is not big enough for the results to be generalizable to the population. Lastly, it is a single-center study conducted at a tertiary care hospital, hence the results might not reflect the true clinical, histological and prognostic picture of the disease in the population.
Conclusion
This is the first report from Pakistan documenting the steroid use for AIN and its effects on renal outcomes in our population. We found poorer renal outcomes in our population stressing the need for earlier diagnosis, vigilant suspicion and low threshold for renal biopsy and withdrawal of suspected offending agent. Our data showed the benefit of earlier use of steroids in achieving rapid and complete renal recovery. It adds to the forever-growing body of evidence in favor of steroid use in AIN but prospective, randomized trials are needed to settle the debate once and for all.
Acknowledgments
None to declare.
Financial Disclosure
No funding was received for this study.
Conflict of Interest
The authors declare that they have no competing interests.
Informed Consent
The requirement of written informed consent was waived due to the retrospective nature of the study.
Author Contributions
AA, SY and MRAR designed this study; AA, AK and MRAR collected the data; SA conducted data analysis and interpretation; AA and SY drafted manuscript; and SA, MRAR and SY revised the manuscript. Each author has read and approved the final version.
Data Availability
The data used in this study are available from the corresponding author on request.
| References | ▴Top |
- Raghavan R, Eknoyan G. Acute interstitial nephritis - a reappraisal and update. Clin Nephrol. 2014;82(3):149-162.
doi pubmed - Goicoechea M, Rivera F, Lopez-Gomez JM, Spanish Registry of G. Increased prevalence of acute tubulointerstitial nephritis. Nephrol Dial Transplant. 2013;28(1):112-115.
doi pubmed - Quinto LR, Sukkar L, Gallagher M. Effectiveness of corticosteroid compared with non-corticosteroid therapy for the treatment of drug-induced acute interstitial nephritis: a systematic review. Intern Med J. 2019;49(5):562-569.
doi pubmed - Das U, Dakshinamurty KV, Prayaga A. Pattern of biopsy-proven renal disease in a single center of south India: 19 years experience. Indian J Nephrol. 2011;21(4):250-257.
doi pubmed - Mubarak M, Kazi JI, Naqvi R, Ahmed E, Akhter F, Naqvi SA, Rizvi SA. Pattern of renal diseases observed in native renal biopsies in adults in a single centre in Pakistan. Nephrology (Carlton). 2011;16(1):87-92.
doi pubmed - Li LS, Liu ZH. Epidemiologic data of renal diseases from a single unit in China: analysis based on 13,519 renal biopsies. Kidney Int. 2004;66(3):920-923.
doi pubmed - Wilson GJ, Kark AL, Francis LP, Hoy W, Healy HG, Mallett AJ. The increasing rates of acute interstitial nephritis in Australia: a single centre case series. BMC Nephrol. 2017;18(1):329.
doi pubmed - Valluri A, Hetherington L, McQuarrie E, Fleming S, Kipgen D, Geddes CC, Mackinnon B, et al. Acute tubulointerstitial nephritis in Scotland. QJM. 2015;108(7):527-532.
doi pubmed - Praga M, Sevillano A, Aunon P, Gonzalez E. Changes in the aetiology, clinical presentation and management of acute interstitial nephritis, an increasingly common cause of acute kidney injury. Nephrol Dial Transplant. 2015;30(9):1472-1479.
doi pubmed - Gonzalez E, Gutierrez E, Galeano C, Chevia C, de Sequera P, Bernis C, Parra EG, et al. Early steroid treatment improves the recovery of renal function in patients with drug-induced acute interstitial nephritis. Kidney Int. 2008;73(8):940-946.
doi pubmed - Blank ML, Parkin L, Paul C, Herbison P. A nationwide nested case-control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int. 2014;86(4):837-844.
doi pubmed - Leonard CE, Freeman CP, Newcomb CW, Reese PP, Herlim M, Bilker WB, Hennessy S, et al. Proton pump inhibitors and traditional nonsteroidal anti-inflammatory drugs and the risk of acute interstitial nephritis and acute kidney injury. Pharmacoepidemiol Drug Saf. 2012;21(11):1155-1172.
doi pubmed - Muriithi AK, Leung N, Valeri AM, Cornell LD, Sethi S, Fidler ME, Nasr SH. Clinical characteristics, causes and outcomes of acute interstitial nephritis in the elderly. Kidney Int. 2015;87(2):458-464.
doi pubmed - Perazella MA. Diagnosing drug-induced AIN in the hospitalized patient: a challenge for the clinician. Clin Nephrol. 2014;81(6):381-388.
doi pubmed - Schwarz A, Krause PH, Kunzendorf U, Keller F, Distler A. The outcome of acute interstitial nephritis: risk factors for the transition from acute to chronic interstitial nephritis. Clin Nephrol. 2000;54(3):179-190.
- Naqvi R, Mubarak M, Ahmed E, Akhtar F, Naqvi A, Rizvi A. Acute tubulointerstitial nephritis/drug induced acute kidney injury; an experience from a single center in Pakistan. J Renal Inj Prev. 2016;5(1):17-20.
doi pubmed - Rossert J. Drug-induced acute interstitial nephritis. Kidney Int. 2001;60(2):804-817.
doi pubmed - Baker RJ, Pusey CD. The changing profile of acute tubulointerstitial nephritis. Nephrol Dial Transplant. 2004;19(1):8-11.
doi pubmed - Laberke HG, Bohle A. Acute interstitial nephritis: correlations between clinical and morphological findings. Clin Nephrol. 1980;14(6):263-273.
- Clarkson MR, Giblin L, O'Connell FP, O'Kelly P, Walshe JJ, Conlon P, O'Meara Y, et al. Acute interstitial nephritis: clinical features and response to corticosteroid therapy. Nephrol Dial Transplant. 2004;19(11):2778-2783.
doi pubmed - Navaratnarajah A, Sambasivan K, Cook TH, Pusey C, Roufosse C, Willicombe M. Predicting long-term renal and patient survival by clinicopathological features in elderly patients undergoing a renal biopsy in a UK cohort. Clin Kidney J. 2019;12(4):512-520.
doi pubmed - Muriithi AK, Leung N, Valeri AM, Cornell LD, Sethi S, Fidler ME, Nasr SH. Biopsy-proven acute interstitial nephritis, 1993-2011: a case series. Am J Kidney Dis. 2014;64(4):558-566.
doi pubmed - Fernandez-Juarez G, Perez JV, Caravaca-Fontan F, Quintana L, Shabaka A, Rodriguez E, Gadola L, et al. Duration of treatment with corticosteroids and recovery of kidney function in acute interstitial nephritis. Clin J Am Soc Nephrol. 2018;13(12):1851-1858.
doi pubmed - Prendecki M, Tanna A, Salama AD, Tam FW, Cairns T, Taube D, Cook HT, et al. Long-term outcome in biopsy-proven acute interstitial nephritis treated with steroids. Clin Kidney J. 2017;10(2):233-239.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Nephrology and Urology is published by Elmer Press Inc.
